
.jpg)
In dry cleaning, solvents other than water are used It is still used today in a limited capacity. Later Fuller’s earth was used by itself, instead of urine. Clays that can absorb oils and other biological materials, known now as Fuller’s earth, were sometimes used together with urine. Because of the unpleasant nature of this work, it was generally done by slaves. In Ancient Rome fulling often involved submerging the wool and fabric in fermented urine and stomping it. Generally, ammonia was derived from animal and human urine, and this urine was in so much demand in Ancient Rome, that its sales were taxed. Historically, ammonium salts dissolved in water were used to clean clothes and wool fabric or to prepare wool for further use in the process of fulling or walking.
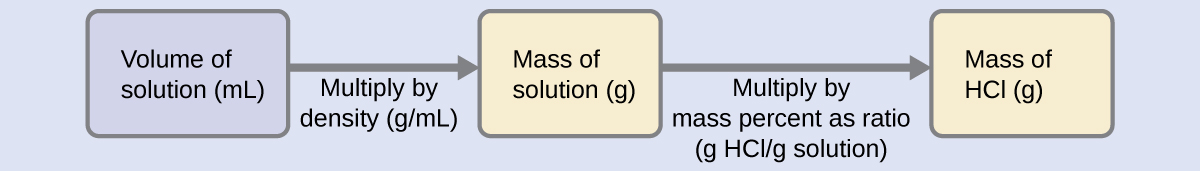
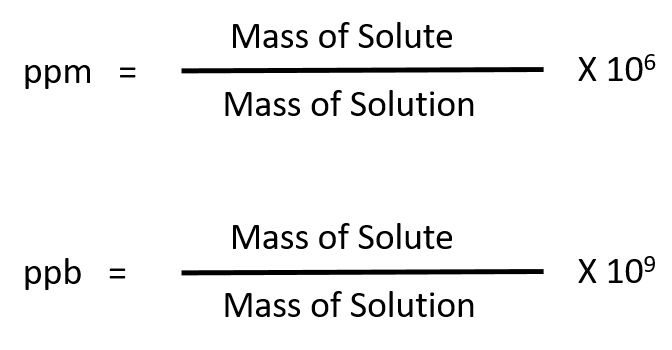
There are also other types of cleaners that “lift” the stain, called emulsifiers, and biological cleaners made from enzymes, which “digest” the stain. The detergent acts as a solvent, and the “dirt” is a solute that dissolves in the solvent. When we clean something, the soiling and the cleaner form a solution. Water is the universal solvent In CleaningĬleaning is a chemical process that involves dissolving the stains or other soiling. Below are some examples of solvents and solutions used in everyday life. Once the solute and the solvent are mixed, their properties change, but the physical state is the same as that of the solvent. Solutions with a high concentration of the solute are called concentrated or saturated, and those with low concentration are referred to as dilute or unsaturated solutions. For most substances temperature increase corresponds to the increase in the amount of this solute that can be diluted, but in some cases, this relationship is the opposite. There is generally a limit on the possible concentration of a solution, but this threshold changes with temperature and pressure. The substance being diluted is called solute. When talking about solutions we say that the substance in which we dilute something is a solvent. Milk and blood are some examples of colloids, while air mixed with dust particles, or seawater mixed during a storm with small sand and dust particles are suspensions. The last type is a suspension - it has even larger particles and can be separated when the particles settle. A colloid, the second type, is similar to a solution but may look opaque or translucent and contains particles that are larger than in a solution. When two or more substances are mixed, three different types of a mixture can occur, and a solution is just one of these. The laser pointer light test demonstrates that there are millions of sub-microscopic particles suspended in this colloid of magenta inkjet ink in water It is easy to convert this value to concentration percentage - we simply multiply the result by 100%. To find mass concentration we divide the mass of the substance that we dilute by the total volume of the resulting substance. In this converter, we consider concentration measured by mass, although volume and percent concentration are also commonly measured. It is important to be able to measure or to adjust the concentration of a solution because different concentrations result in solutions with different properties. Some drinks, as well as many other substances, are also solutions. Many household cleaners and chemicals are solutions or form solutions with the dirt. Solutions are used widely in medicine, cosmetics, cooking, painting, and industrial cleaning. Not every mix can be called a solution - only those that cannot be separated mechanically, and are in a uniform state (for example, all liquid) are solutions. We often use solutions - substances that are formed by mixing several other substances. In everyday life as well as in the industry we rarely use pure substances - even water has different elements mixed into it unless it is distilled. 1 - sugar (solute), 2 - water (solvent), 3 - sugar solution


 0 kommentar(er)
0 kommentar(er)
